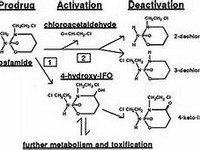
dobutamine

dobutamine
CLINICAL USE
Inotropic agentDOSE IN NORMAL RENAL FUNCTION
2.5–10 micrograms/kg/minute, increasing up to 40 micrograms/kg/minute according to responsePHARMACOKINETICS
DOSE IN RENAL IMPAIRMENT
GFR (mL/MIN)
DOSE IN PATIENTS UNDERGOING RENAL REPLACEMENT THERAPIES
IMPORTANT DRUG INTERACTIONS
Potentially hazardous interactions with other drugsBeta-blockers: possibly severe hypotension with beta-blockersDopaminergics: effects possibly enhanced by entacapone; avoid concomitant use with rasagilineADMINISTRATION
Reconstition
–Route
ContinuousIV infusion
centrally via CRIP (or peripherally via a large vein)Rate of Administration
Varies with doseComments
Dilute to at least 50 mL with sodium chloride 0.9% or glucose 5% (less than 5 mg/mL, ideally 0.5–1 mg/mL)250 mg may be diluted in as little as 50 mL diluentMinimum volume 10 mg/mL or even undiluted; give strong solution via central line (UK Critical Care Group, Minimum Infusion Volumes for fluid restricted critically ill patients, 3rd Edition, 2006)OTHER INFORMATION
Cardiac and BP monitoring advised Sodium bicarbonate rapidly inactivates dobutamineSolution may turn pink, but potency is unaffectedCan cause hypokalaemia
See how to identify renal failure stages according to GFR calculation
See how to diagnose irreversible renal disease
Home