Cycloserine
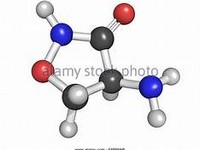
Cycloserine
CLINICAL USE
Antibacterial agent:TuberculosisDOSE IN NORMAL RENAL FUNCTION
Initially 250 mg every 12 hours for 2 weeks; then increased according to blood concentration and response to maximum 500 mg every 12 hoursPHARMACOKINETICS
DOSE IN RENAL IMPAIRMENT
GFR (mL/MIN)
DOSE IN PATIENTS UNDERGOING RENAL REPLACEMENT THERAPIES
IMPORTANT DRUG INTERACTIONS
Potentially hazardous interactions with other drugsAlcohol: Increased risk of seizuresADMINISTRATION
Reconstition
–Route
OralRate of Administration
–Comments
_OTHER INFORMATION
May cause drowsiness – increased cerebral sensitivity in patients with renal impairmentBlood concentration monitoring is required, especially in renal impairment, if dose exceeds 500 mg daily, or if signs of toxicity. Blood concentration should not exceed 30 mg/LContraindicated in severe renal insufficiencyCan cause CNS toxicity Pyridoxine has been used in an attempt to treat or prevent neurological reactions, but its value is unproven.
See how to identify renal failure stages according to GFR calculation
See how to diagnose irreversible renal disease
Home