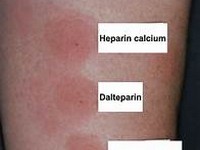
Fluorouracil
Fluorouracil
CLINICAL USE
Antineoplastic agentDOSE IN NORMAL RENAL FUNCTION
IV infusion
: 15 mg/kg/day to a total dose of 12–15 gIV bolus: 12 mg/kg/day for 3 days, then 6 mg/kg on alternate days or 15 mg/kg once a weekMaintenance: 5–15 mg/kg once a week Intra-arterial infusion: 5–7.5 mg/kg by continuous 24-hour infusionOral: 15 mg/kg weekly; maximum 1 g in a dayOr consult relevant local chemotherapy protocolPHARMACOKINETICS
DOSE IN RENAL IMPAIRMENT
GFR (mL/MIN)
DOSE IN PATIENTS UNDERGOING RENAL REPLACEMENT THERAPIES
IMPORTANT DRUG INTERACTIONS
Potentially hazardous interactions with other drugsADMINISTRATION
Reconstition
Consult relevant local protocolRoute
IV infusion
intermittent or continuous, IV injection, intra-arterial, oral, topicalRate of Administration
30–60 minutes, 4 hours or as a continuous infusion over 24 hours or consult relevant local protocolComments
–OTHER INFORMATION
Use ideal body weight in patients showing obesity, ascites, and oedemaRoche recommends decreasing the initial dose by one-third to one-half in patients with impaired hepatic or renal functionDistributed throughout the body water, activated in target cells, most of dose (80%) is metabolised by the liver, 60–80% is excreted as respiratory CO2 and 2–3% by the biliary systemFollowing a single IV dose, approximately 15% is excreted unchanged in the urine.
See how to identify renal failure stages according to GFR calculation
See how to diagnose irreversible renal disease
Home