Carmustine
Carmustine
CLINICAL USE
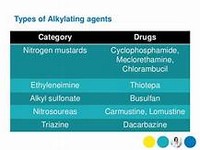
Alkylating agent:
Myeloma, lymphoma and brain tumours DOSE IN NORMAL RENAL FUNCTION
150–200 mg/m2 as a single dose or 75–100 mg/m2 on 2 consecutive days every 6 weeks Implants: 7.7mg, maximum 8 implants PHARMACOKINETICS
Molecular weight :214.1 %Protein binding :77 %Excreted unchanged in urine : 60–70 Volume of distribution (L/kg) :3.25half-life – normal/ESRD (hrs) :22 minutes/– DOSE IN RENAL IMPAIRMENT
GFR (mL/MIN)
20 to 50 : Dose as in normal renal function 10 to 20 : Dose as in normal renal function <10 : Dose as in normal renal function DOSE IN PATIENTS UNDERGOING RENAL REPLACEMENT THERAPIES
CAPD :Not dialysed. Dose as in normal renal function HD :Not dialysed. Dose as in normal renal functionHDF/high flux :Unknown dialysability. Dose as in normal renal functionCAV/VVHD :Not dialysed. Dose as in normal renal function IMPORTANT DRUG INTERACTIONS
Potentially hazardous interactions with other drugsNone known ADMINISTRATION
Reconstition
3 mL of the supplied diluent (absolute ethanol) then add 27 mL of sterile water for injectionThis solution may be further diluted with sodium chloride 0.9% or glucose 5% for injection Route
IV Rate of Administration
Administer by IV drip over a period of 1–2 hoursComments
Therapy should not be repeated before 6 weeksCan further dilute the reconstituted solution with 500 mL of sodium chloride 0.9% or glucose 5% OTHER INFORMATION
Renal abnormalities, e.g. a decrease in kidney size: progressive azotaemia and renal failure have been reported in patients receiving large cumulative doses after prolonged therapyPartially metabolised to active species by liver microsomal enzymes, which have a long T½. It is thought that the antineoplastic activity may be due to metabolites. Approximately 30% of a dose is excreted in the urine after 24 hours, and 60–70% of the total dose after 96 hours. About 10% is excreted as respiratory CO2. Terminal half-life of the metabolites are about 1 hour.
See how to identify renal failure stages according to GFR calculation
See how to diagnose irreversible renal disease
Home