ifosfamide
CLINICAL USE
Antineoplastic agent:Treatment of solid tumours, lymphomas and soft tissue sarcoma
DOSE IN NORMAL RENAL FUNCTION
Usual total dose for each course is either 8–12 g/m2, equally divided as single daily doses over 3–5 days, or 5–6 g/m2 (maximum 10 g) given as a 24 hour infusionOr according to local protocol
PHARMACOKINETICS
DOSE IN RENAL IMPAIRMENT
GFR (mL/MIN)
>60 80% of normal dose30–60 80% of normal dose15–30 80% of normal dose<15 60% of normal dose
DOSE IN PATIENTS UNDERGOING RENAL REPLACEMENT THERAPIES
IMPORTANT DRUG INTERACTIONS
Potentially hazardous interactions with other drugs
ADMINISTRATION
Reconstition
Reconstitute 1 g vial with 12.5 mL water for injection. Reconstitute 2 g vial with 25 mL water for injection. The resultant solution of 8% ifosfamide should NOT be injected directly into the vein
Route
IV injection: dilute to less than a 4% solution
IV infusion
: dilute as detailed below
Rate of Administration
IV infusion
: Infuse in glucose 5% or sodium —chloride 0.9% over 30–120 minutes, or Inject directly into a fast running —infusion, orMade up in 3 L of glucose 5% or —sodium chloride 0.9%; each litre should be given over 8 hours
Comments
–
OTHER INFORMATION
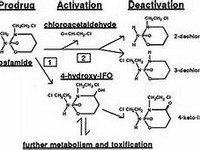
Nephrotoxicity may occur with oliguria, raised uric acid, increased BUN and serum creatinine, and decreased creatinine clearanceIfosfamide is known to be more nephrotoxic than cyclophosphamide; hence greater caution is advisedSPC contraindicates the use of ifosfamide if serum creatinine >120 µmol/LIf patient is anuric and on dialysis, neither the ifosfamide nor its metabolites nor mesna should appear in the urinary tract. The use of mesna may therefore be unnecessary, although this would be a clinical decisionIf the patient is passing urine, mesna should be given to prevent urothelial toxicityIfosfamide is a prodrug – converted by hepatic microsomal enzymes to alkylating metabolites. Excretion is primarily renal. Approximately 80% of dose is excreted as parent compound.372 iFosFAMidEDoses from Kintzel PE, Dorr RT. Anticancer drug renal toxicity and elimination: dosing guidelines for altered renal function. Can Treat Rev. 1995; 21: 33–64:GFR > 60 mL/min 80% of doseGFR = 45–60 mL/min 75% of doseGFR = 30–45 mL/min 70% of dose