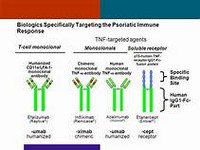
Efalizumab
CLINICAL USE
Treatment of patients with moderate to severe chronic plaque psoriasis, who have failed to respond to other therapies or who are unable to take other therapies
DOSE IN NORMAL RENAL FUNCTION
Initially: 0.7 mg/kg followed by weekly injections of 1 mg/kg (maximum single dose should be <200 mg) for 12 weeks
PHARMACOKINETICS
DOSE IN RENAL IMPAIRMENT
GFR (mL/MIN)
DOSE IN PATIENTS UNDERGOING RENAL REPLACEMENT THERAPIES
IMPORTANT DRUG INTERACTIONS
Potentially hazardous interactions with other drugsImmunosuppressants: may potentiate the effect of immunosuppressantsVaccines: do not administer live vaccines during treatment
ADMINISTRATION
Reconstition
With solvent provided (water for injection)
Route
SC
Rate of Administration
–
Comments
–
OTHER INFORMATION
Use with caution in patients with a history of recurrent infections